Epistatic interactions influence terrestrial–marine functional shifts in cetacean rhodopsin

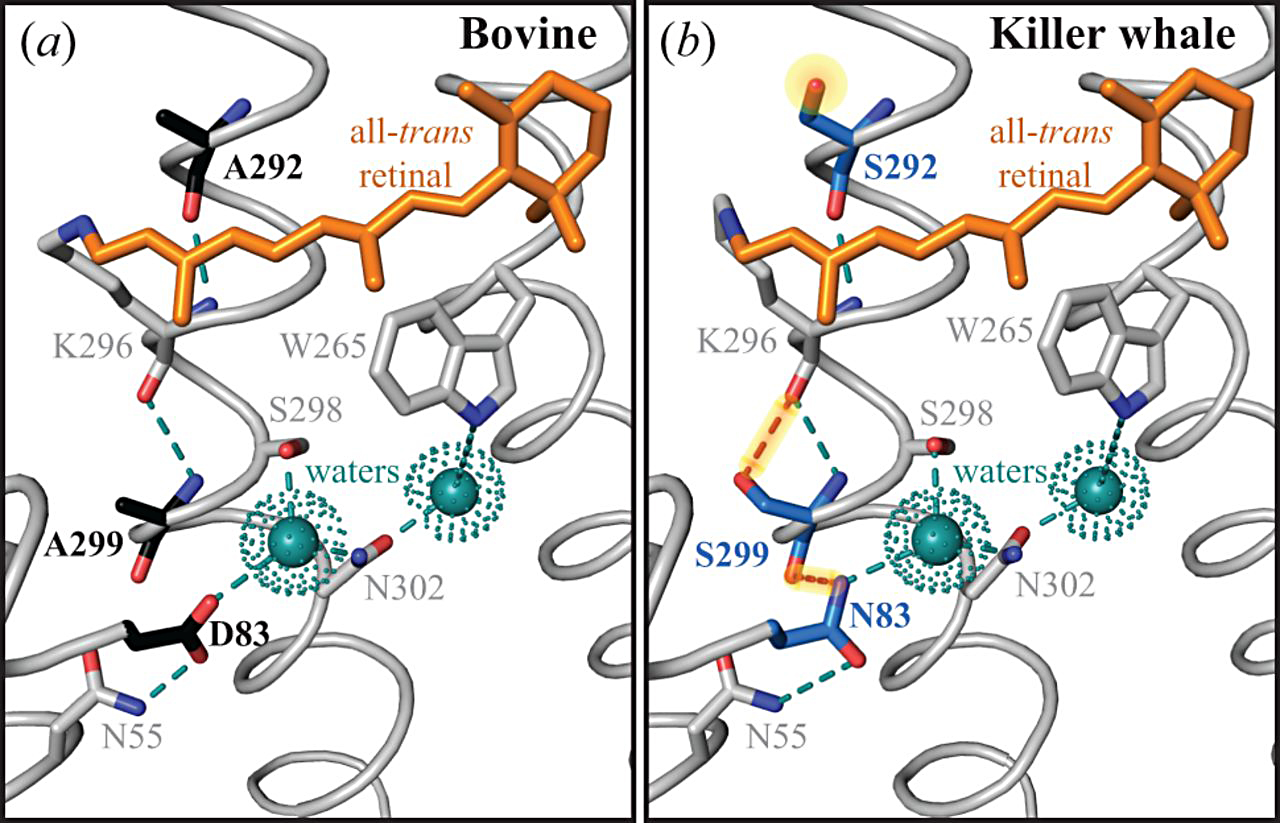
PhD candidate, Sarah Dungan, and Professor Belinda Chang from EEB recently published results on the visual protein, rhodopsin, from the killer whale, a cetacean. They provide the first experimental results for light-activation kinetics in a cetacean visual pigment. Two mutations which cause a blue-shift to spectral sensitivity in the cetacean rhodopsin also alter kinetic rates in a model system (bovine rhodopsin), but not the killer whale. This result points to intramolecular epistasis. Ancestral rhodopsin sequence reconstruction also supports the killer whale pigment as being derived with respect to spectral sensitivity, but similar to an ancestral outgroup (hippopotamus) with respect to activation kinetics.
See Press Release
Context matters for evolution at the molecular level Mar1/2017
Abstract
Like many aquatic vertebrates, whales have blue-shifting spectral tuning substitutions in the dim-light visual pigment, rhodopsin, that are thought to increase photosensitivity in underwater environments. We have discovered that known spectral tuning substitutions also have surprising epistatic effects on another function of rhodopsin, the kinetic rates associated with light-activated intermediates. By using absorbance spectroscopy and fluorescence-based retinal release assays on heterologously expressed rhodopsin, we assessed both spectral and kinetic differences between cetaceans (killer whale) and terrestrial outgroups (hippo, bovine). Mutation experiments revealed that killer whale rhodopsin is unusually resilient to pleiotropic effects on retinal release from key blue-shifting substitutions (D83N and A292S), largely due to a surprisingly specific epistatic interaction between D83N and the background residue, S299. Ancestral sequence reconstruction indicated that S299 is an ancestral residue that predates the evolution of blue-shifting substitutions at the origins of Cetacea. Based on these results, we hypothesize that intramolecular epistasis helped to conserve rhodopsin’s kinetic properties while enabling blue-shifting spectral tuning substitutions as cetaceans adapted to aquatic environments. Trade-offs between different aspects of molecular function are rarely considered in protein evolution, but in cetacean and other vertebrate rhodopsins, may underlie multiple evolutionary scenarios for the selection of specific amino acid substitutions.
Published on March 01, 2017 in the scientific journal, Proceedings of the Royal Society B: Biological Sciences
https://royalsocietypublishing.org/doi/full/10.1098/rspb.2016.2743
